Abstract
Introduction: Rituximab as monotherapy or in combination with chemotherapy has been the mainstay for treatment of indolent B-cell non-Hodgkin lymphoma (iNHL) in clinical practice for 25 years. Patients with disease who progress <6 months from prior rituximab treatment are considered refractory and may be best treated with a treatment modality other than the initial one. Patients progressing >12 months from prior rituximab treatment are considered rituximab-sensitive and may be candidates for retreatment either with rituximab monotherapy or with arituximab-based combination. To gain a better understanding of rituximab use in clinical practice in patients with relapsed iNHL, rituximab prescriptions from two large national-level health claims databases were analyzed and rituximab usage in patients with a treatment-free interval of a minimum of 180 days between the end of the first treatment and the start of the second treatment was identified.
Methods: This is a retrospective cohort study based on 2 large US claims databases: Optum Clinformatics (DB1) and Marketscan Commercial (DB2). Adult patients (≥18 years old) with at least one claim for a diagnosis of B-cell NHL (FL, MZL, SLL, WM/LPL) between July 1, 2007 and January 1, 2020 were identified using ICD-9 and ICD-10 codes. Patients had to have continuous insurance eligibility with medical and pharmacy benefit coverage from 12 months prior to diagnosis of iNHL until at least 90 days after the start of the second treatment with rituximab monotherapy or combination. Relapse was defined as a treatment-free period of at least 180 days between the end of the first rituximab-based treatment and the start of the second rituximab-based treatment. Patients were categorized into two groups based on whether patients received rituximab as monotherapy or as combination therapy after relapse.
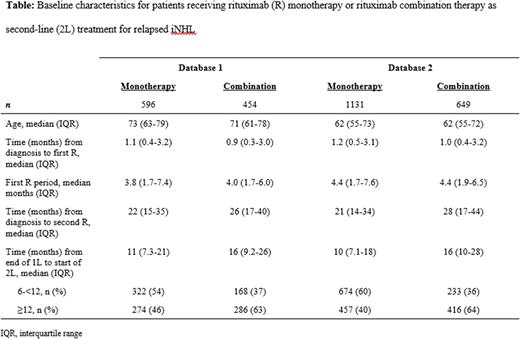
Results: From July 2007 to January 2020, a total 13,037 patients from DB1 and 23,590 patients from DB2 were diagnosed with iNHL and received first rituximab-based treatment, 3731 (29%) and 7153 (30%), respectively, as monotherapy and 9306 (71%) and 16,437 (70%) in combination. Of those receiving first rituximab-based treatment, 1050 (28%) patients from DB1 had a treatment-free interval of ≥180 days from the end of the first rituximab-based treatment to the start of the second rituximab-based treatment, of whom 596 (57%) received rituximab as monotherapy and 454 (43%) in combination. Similarly, of those receiving first rituximab-based treatment in DB2, 1780 (25%) patients had a treatment-free interval of ≥180 days from the end of the first rituximab-based treatment to the start of the second rituximab-based treatment, of whom 1131 (64%) received rituximab monotherapy and 649 (36%) in combination. Further exploration of patient characteristics for patients receiving rituximab monotherapy as retreatment for relapsed iNHL are shown in Table. Patients were slightly older in DB1, but otherwise the treatment patterns were similar for both databases. Patients receiving rituximab combination therapy as retreatment had a longer treatment-free interval compared to patients receiving rituximab monotherapy after relapse in both databases. Likewise, patients with a treatment-free interval of >6 month but <12 months were more likely to receive rituximab monotherapy after relapse, whereas those with a treatment-free interval of ≥12 months were more likely to receive rituximab-based combination.
Conclusions: The rituximab treatment patterns for relapsed iNHL were markedly consistent across two large US claims databases despite differences in the two commercially insured populations. Based on these RWD, rituximab combinations were more frequently prescribed as initial treatment, while rituximab monotherapy was more commonly prescribed in the relapsed setting. However, in the relapsed setting, if the treatment-free interval was >12 months, then rituximab-based combinations were more frequently prescribed. This study provides useful insights for clinicians into real-world treatment patterns. Future research should examine clinical response to various rituximab-based treatments in relapsed setting.
Disclosures
Said:Bayer: Current Employment. Khan:Bayer: Current Employment. Zong:Bayer: Current Employment. Chen:Bayer: Current Employment. Healy:Bayer: Current Employment. Reddy:Bayer: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal